Platinum is a noble metal and is and is one of the most expensive precious metal together with rhodium and gold. It has the face centered cubic crystal structure. At room temperature, platinum is soft (HV 55) and very ductile. It can be cold worked to more than 90 % deformation by rolling and drawing. The undeformed metal has a Young’s modulus of 165 GPa.
Despite its high melting point of about 1,769°C, platinum recrystallizes at a temperature of approx. 525°C.
The mechanical strength of platinum is increased by additions of alloying elements such as rhodium or iridium. The strength at temperatures >1,200°C can also increase by introducing small, finely divided oxide particles into the microstructure (oxide dispersion hardened).
Platinum is resistant to most types of molten glass and is insoluble in acids. It only dissolves in hot hydrochloric acid containing an oxidizing agent, e.g. HNO3 (classical aqua regia) or elemental chlorine, to hexachloroplatinic(IV) acid, H2Cl6Pt · 6H2O:
8HCl + 2HNO3 + Pt → H2[PtCl6] + 4H2O + 2NOCl
Platinum is attacked by molten alkalis, cyanides and many molten salts. Above 1,200 °C it is the most oxidation resistant metal, but oxidizes slowly in air to the volatile oxides platinum monoxide, PtO, and platinum dioxide, PtO2. As a result of its resistance to chemical changes, the physical properties of platinum (mass, volume, electrical resistance, electromotive force) remain constant over very long periods of time.
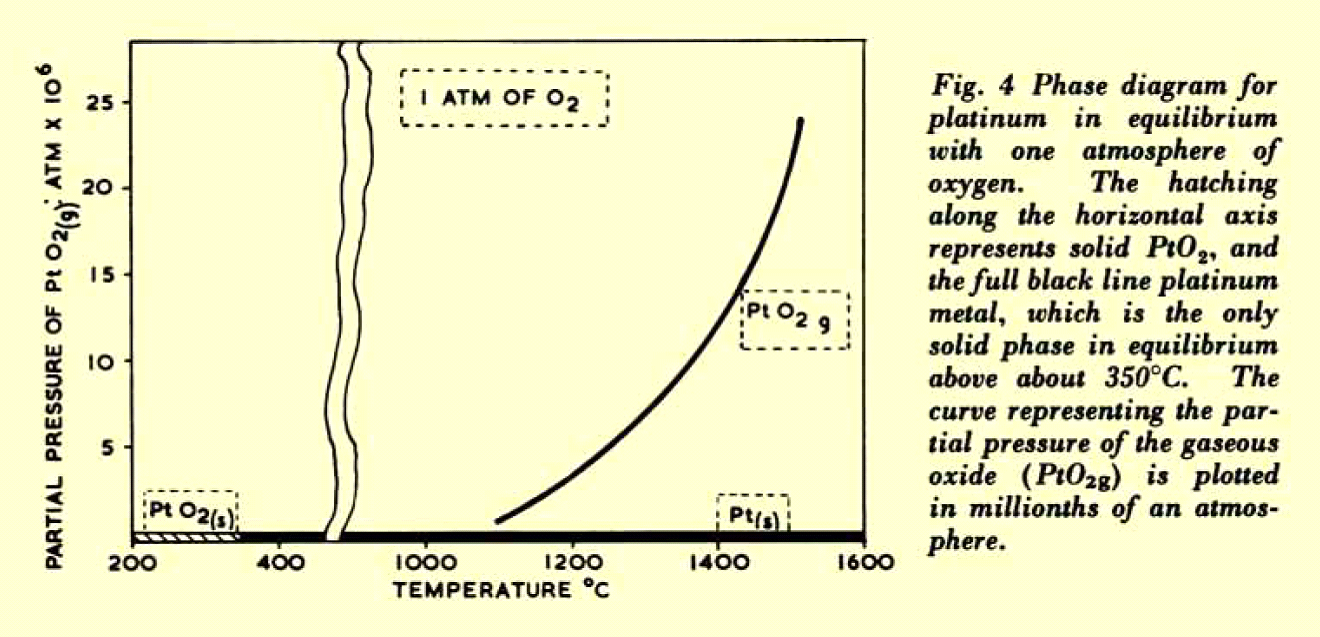
Source: Platinum Metals Review, Vol. 9, 1965, p. 51